Apparatus 7 (Reciprocating Holder)
The assembly consists of (1) a set of volumetrically calibrated or tared solution containers made of glass or other suitable inert material that should not sorb, react with, or interfere with the specimen being tested; (2) a motor and drive assembly to reciprocate the system vertically and to index the system horizontally to a different row of vessels, automatically if desired; and (3) a set of suitable sample holders (see Figure 4 and Figures 5a, 5b, 5c, and 5d).
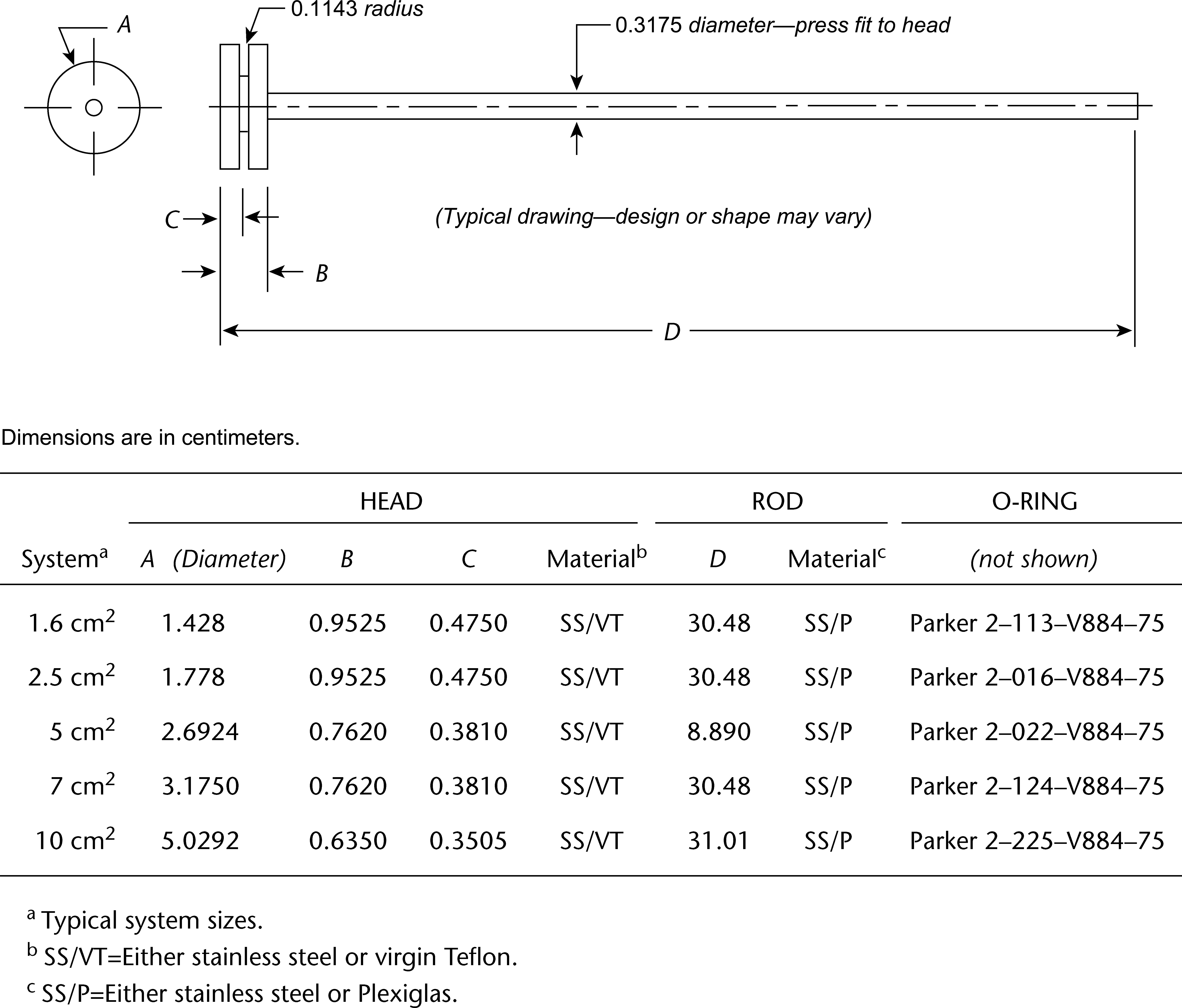
Figure 4. Reciprocating disk sample holder.
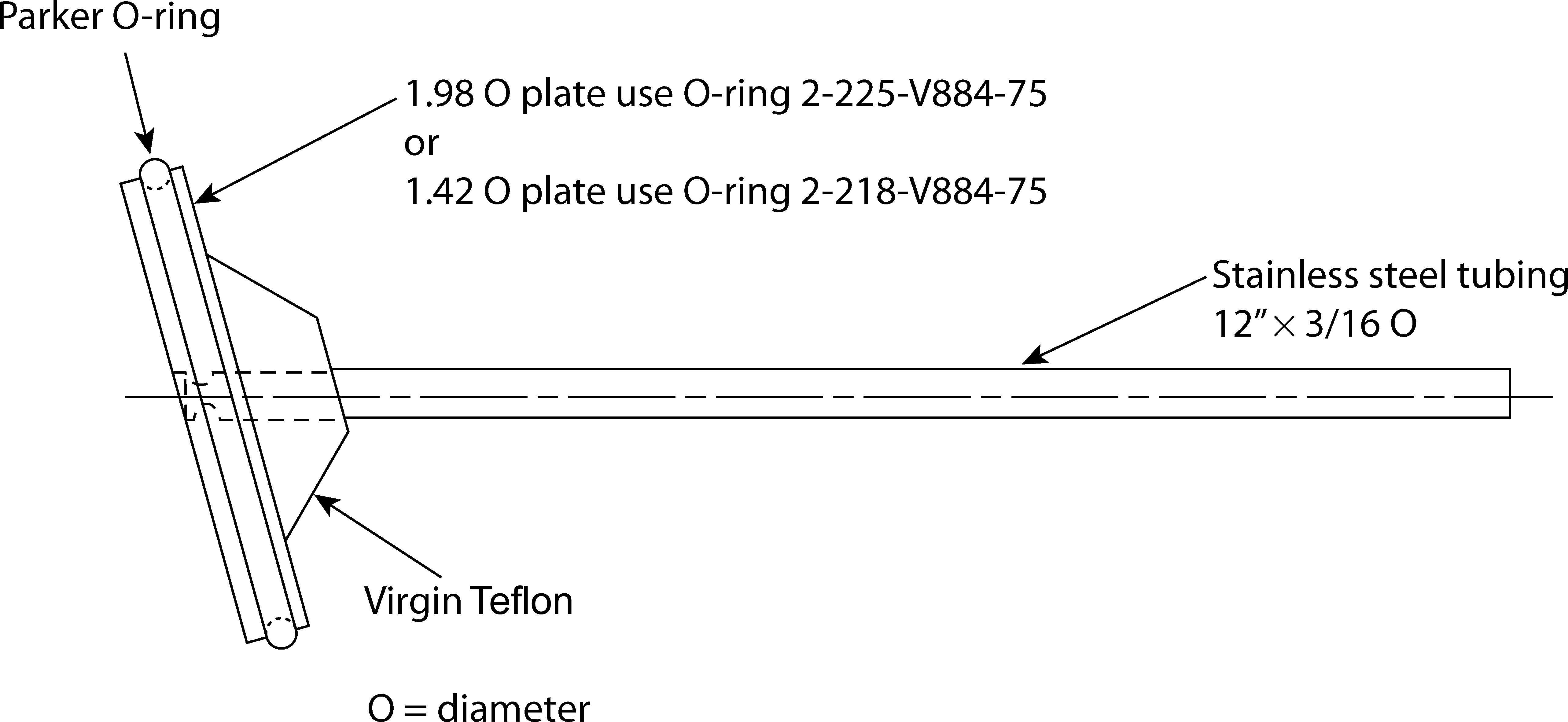
Figure 5a. Transdermal system holder—angled disk.
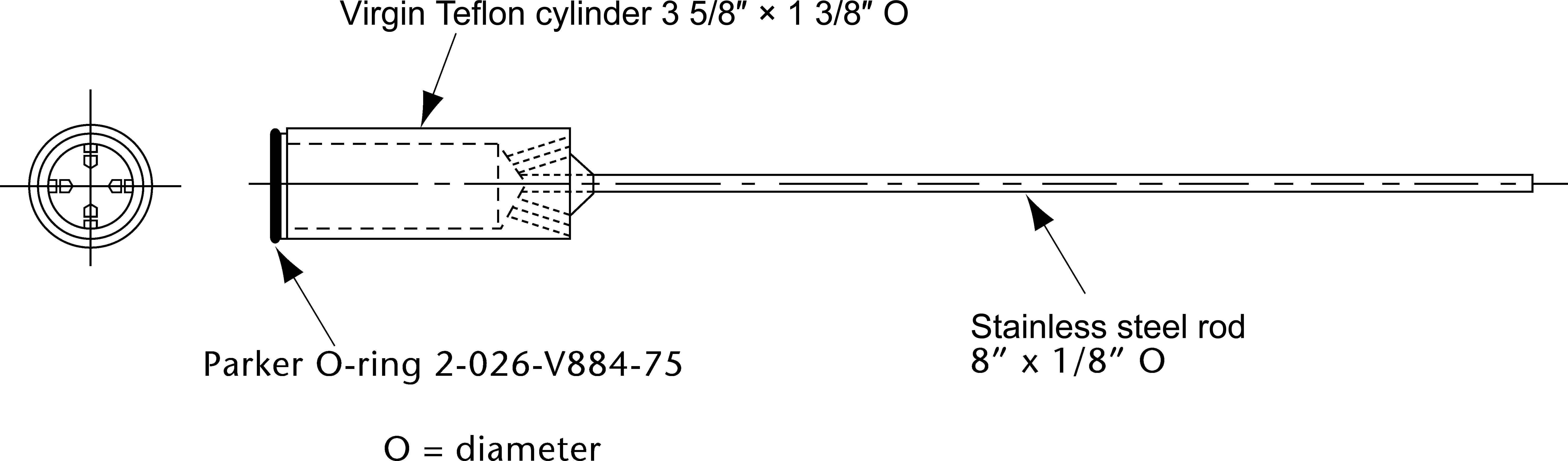
Figure 5b. Transdermal system holder—cylinder.
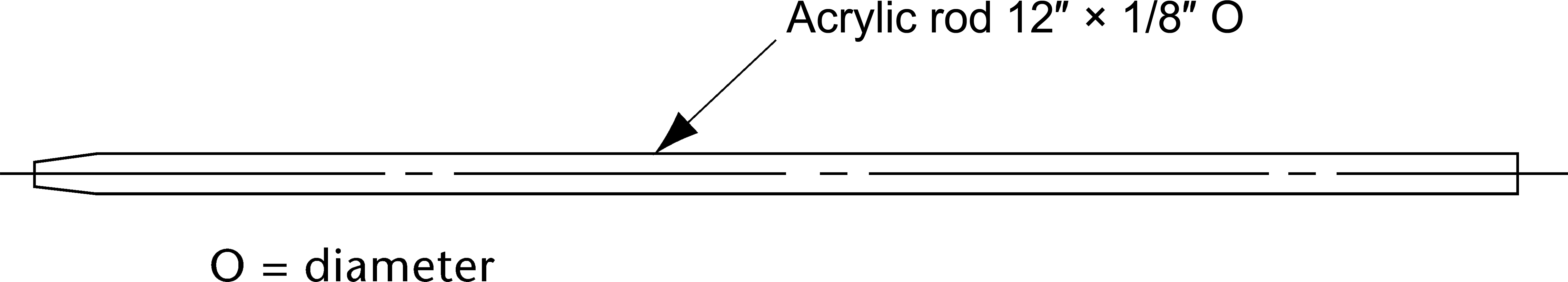
Figure 5c. Oral extended-release tablet holder—rod, pointed for gluing.
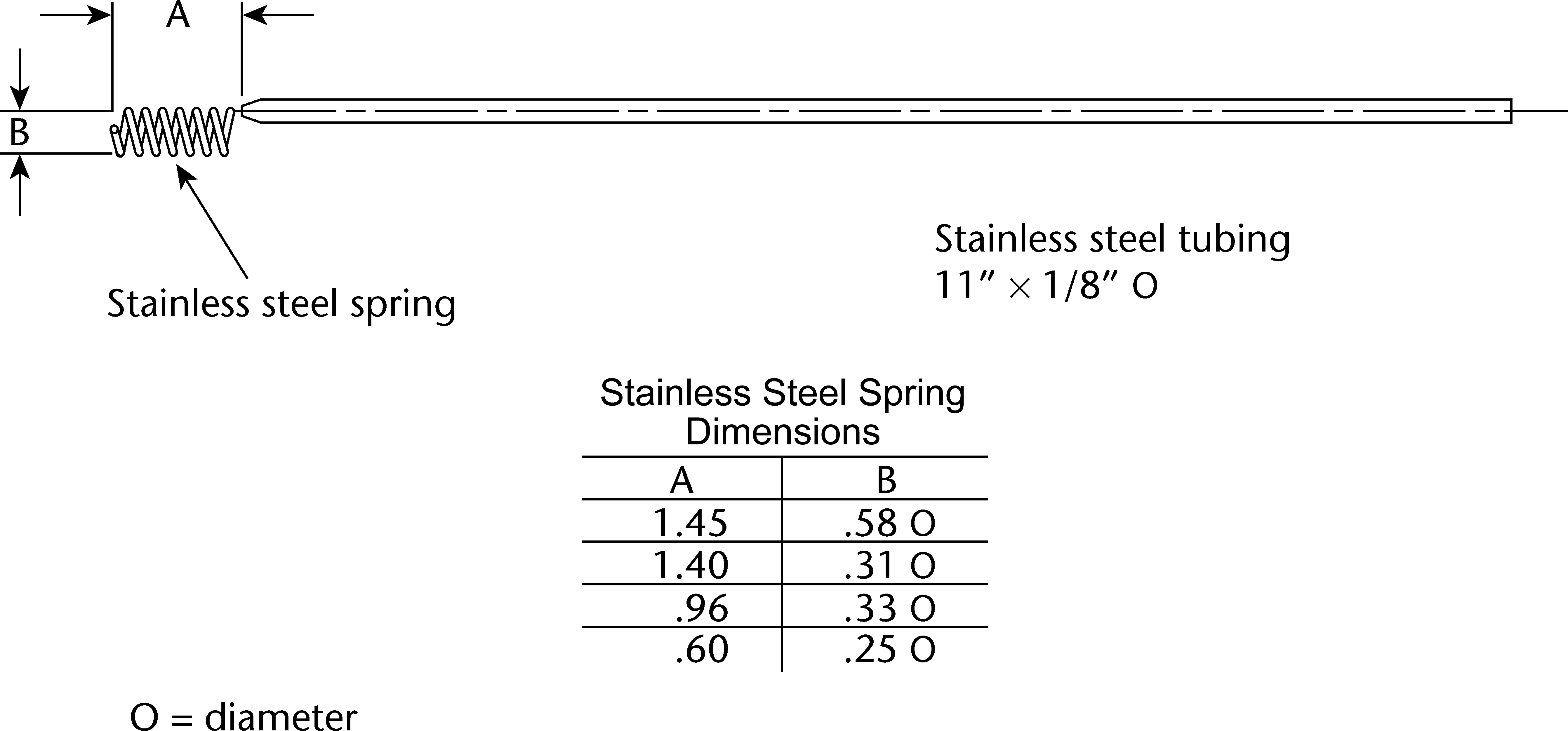
Figure 5d. Oral extended-release tablet holder—spring holder.
During the test, the solution containers are partially immersed in a suitable water bath of any convenient size that permits maintaining the temperature inside the containers at 32 ± 0.5° for TDS or at 37 ± 0.5° for other dosage forms during the test.
No part of the assembly, including the environment in which the assembly is placed, contributes significant motion, agitation, or vibration beyond that due to the smooth, vertically reciprocating sample holder.
Apparatus that permits observation of the system and holder during the test is preferable.
Use the size container and sample holder as specified in the individual monograph.
MEDIUM
See Dissolution Medium in Dissolution á711ñ, Procedure, Apparatus 1 and Apparatus 2, Immediate-Release Dosage Forms.
SAMPLE PREPARATION A (FOR OSMOTIC PUMP TABLETS)
Attach each unit to be tested to a suitable holder by an appropriate and validated procedure such as use of an adhesive to
adhere the edge of the tablet to a holder (e.g., Figure 5c), the spring holder (Figure 5d), a small nylon net bag, or a membrane.
SAMPLE PREPARATION B (FOR TDS)
Apply the TDS to the appropriate holder, assuring that the release surface is as smooth as possible and the TDS is completely and firmly attached to the holder. The TDS, with release side facing the Medium, may be attached to the holder by an appropriate and validated procedure such as use of an adhesive, double-face adhesive tape, membrane, or nylon net. Care must be taken to avoid the presence of air bubbles between the membrane, if used, and the TDS, or the presence of wrinkles on the surface of the TDS. Carefully remove the protective liner from the TDS without causing damage to the surface of the TDS. If additional reinforcement of the TDS to the holder is needed, inert metal wire or a polymer ring may be used.
SAMPLE PREPARATION C (FOR OTHER DOSAGE FORMS)
Attach each dosage form to be tested to a suitable holder.
PROCEDURE
Place the stated volume of Medium in the solution containers, and equilibrate to the test temperature.
Suspend each prepared sample holder from a vertically reciprocating shaker such that each system is continuously immersed in Medium during the entire test.
Reciprocate at a frequency of about 30 cycles/min with an amplitude of about 2 cm, or as specified in the individual monograph, for the specified time.
At each sampling time interval, remove the solution containers from the bath, cool to room temperature, and add sufficient solvent (i.e., water in most cases) to correct for evaporative losses.
Perform the analysis on each sample as directed in the individual monograph. Repeat the test with additional transdermal systems, as needed.
TIME
The test time points, at least three, are expressed in hours. Specimens are to be withdrawn within a tolerance of ±15 min or ±2% of the stated time, selecting the tolerance that results in the narrowest time interval.
INTERPRETATION
Unless otherwise specified in the individual monograph, the requirements are met if the quantities of active ingredient released from the TDS conform to Acceptance Table 1 above, or the appropriate acceptance table in Dissolution á711ñ for other dosage forms. Continue testing through the three levels unless the results conform at either L1 or L2 .




