

Functional


Using the Same Lifting Column as High-End Medical Equipment
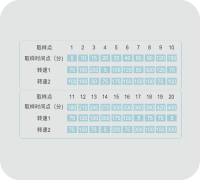
Variable Speed Process

Accessory Stand
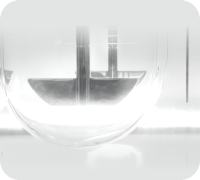
Imaging System

Secondary Filtering

Drain Pan
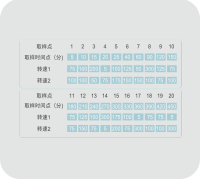
Easy Switch between Accessories
Safety Compliance
Accuracy and Reliability
Highly chemically stable Teflon piping makes every piping channel reliable.
Extensive Functions
Double-zone control is standard, which can signifcantly improve the eficiency of drug quality control and new drug development.
The changeable speed function for dissolution experiments is available to complete the end-point dissolution experiment.
The easy-to-use secondary filtration system greatly improves the efficiency of experiments.
Quickly switch between basket,paddle, and other methods.
Equipped with a dissolution accessory holder and a cleaning tray to facilitate easy storage and cleaning.
Equipped with the same temperature replenishment module as standard to minimize temperature variations after replenishment to guarantee that accurate data can be obtained from the experiment.
With the design of supplemental light, the drug dissolution process can be observed even in poor lighting conditions.
The optional camera system is available to accurately record the dissolution process and the operation status of the instrument.
Technical Specifications
| 12 Position Dissolution Apparatus | Autosampler |
|---|---|
| Temperature control range: room temperature to 50℃ | Test tube/liquid phase vial: 240 positions |
| Temperature control accuracy: ±0.2℃ | Sampling collector size: 450mm×718mm×790mm |
| Main unit size: 910mm×540mm×670mm | Sampling path: 12 ways |
| Dissolution station: 12 positions | Sampling range: 0.5mL ~ 10mL |
| Water tank volume: 46L | Sampling accuracy: ±0.1mL |
| Temperature resolution: 0.1℃ | Maximum sampling times: 20 times |
